
|

Sponsored by
The Pharmaceutical
Compliance Forum
|
SIXTEENTH ANNUAL PHARMACEUTICAL REGULATORY AND COMPLIANCE CONGRESS
Transformational Learning - Effective Knowledge Exchange
October 21 - 23, 2015
Mandarin Oriental
Washington, DC
|
|
KEYNOTE SPEAKERS
|

Thomas W. Abrams, RPh, MBA
Director, Office of Prescription Drug Promotion, Food and Drug Administration, Silver Spring, MD |

Robert Barrington, PhD
Executive Director, Transparency International-UK, London, UK |

Douglas Brown
Deputy Director, Data Sharing & Partnership Group, Center for Program Integrity, Centers for Medicare and Medicaid Services, US Department of Health and Human Services, Baltimore, MD
|

Benjamin C. Mizer, Esq.
Principal Deputy Assistant Attorney General, Civil Division, Former Counselor to Attorney General Eric H. Holder, Jr., US Department of Justice, Washington, DC |

Mary E. Riordan, Esq.
Senior Counsel, Office of Counsel to the Inspector General, Office of Inspector General, US Department of Health and Human Services, Washington, DC
|

Jeffrey S. Sallet
National Chief of Public Corruption and Civil Rights, Federal Bureau of Investigation, Washington, DC
|
|
|
|
FEATURING A COMPLIANCE OFFICER ROUNDTABLE
|

Melissa S. Barnes, Esq.
Chief Ethics and Compliance Officer, Lilly, Indianapolis, IN |

Michael B. Dusseau
Vice President, Compliance Operations, Allergan PLC, Former Vice President, Compliance, Bayer, Parsippany, NJ |

Tricia Glover
Vice President, North America Chief Compliance Officer, Teva Pharmaceuticals, Kansas City, MO
|

Lawrence P. Platkin
Vice President and Compliance Officer, Bayer HealthCare LLC, Whippany, NY |

Trudy Tan
Vice President Compliance North America, AstraZeneca, Wilmington, DE |

Erinn Hutchinson
Partner, PwC, Philadelphia, PA (Moderator)
|
|
|
|
A QUI TAM ROUNDTABLE
|

Jillian Estes, Esq.
Partner, James Hoyer Newcomer & Smiljanich, PA, Former Case Assistant, International Division, National Center for Missing and Exploited Children, Tampa, FL |

Loren Jacobson, Esq.
Partner, Waters & Kraus LLP, Dallas, TX
|

Joseph Trautwein, Esq.
Joseph Trautwein & Associates LLC, Former Assistant US Attorney, Eastern District of Pennsylvania, Philadelphia, PA |

Jonathan L. Diesenhaus, Esq.
Partner, Hogan Lovells LLP, Former Senior Trial Attorney, Civil Fraud Section, US Department of Justice, Washington, DC (Moderator)
|
|
|
|
AN AUSA ROUNDTABLE
|

Charlene Keller Fullmer, Esq.
Assistant United States Attorney, US Attorney's Office, Eastern District of Pennsylvania, Philadelphia, PA |

Jeffrey Steger
Assistant Director, Consumer Protection Branch, Civil Division, U.S. Department of Justice, Washington, DC
|

Kristen Williams, Esq.
Assistant United States Attorney, US Attorney's Office, Los Angeles, CA |

John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP; Former Special Counsel for Healthcare Fraud and Chief Privacy Officer, US Department of Justice, Washington, DC (Moderator)
|
|
|
|
FCPA HYPOTHETICAL CASE STUDY
|

Jeffrey H. Knox, Esq.
Partner, Simpson Thacher & Bartlett LLP; Former Chief, Fraud Section, Criminal Division, Former Assistant U.S. Attorney, Eastern District of New York, US Department of Justice, Washington, DC |

Cheryl J. Scarboro, Esq.
Partner, Simpson Thacher & Bartlett LLP; Former first Chief, FCPA Unit, US Securities and Exchange Commission, Washington, DC
|

Ashley Watson, Esq.
Senior Vice President, Chief Ethics & Compliance Officer, Merck, Inc., Former Counsel, Chief Ethics and Compliance Officer, Hewlett-Packard, Philadelphia, PA |

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings, Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY (Moderator)
|
|
|
|
PRECONFERENCE SESSIONS
|
|
|
- Preconference I: Regulatory Concepts: Basic Principles
- Preconference II: Innovations in Risk Assessment, Auditing and Monitoring
- Preconference III: Advanced Issues in Domestic and Global Transparency and Analytics: Taking the Data to the Next Level
- Preconference IV: Advanced Global Compliance Issues
|
|
PLENARY SESSIONS
|
|
|
- Keynote: OIG Update
- AUSA Panel
- Keynote: FBI's New Focus on FCPA Investigations
- Keynote: FDA-OPDP Update
- Chief Compliance Officer Panel: Now that the CIA is Completed, How is the Ethics and Compliance Program Evolving?
- FCPA Anticorruption Panel: Examining a Hypothetical Case from All Sides: DOJ, SEC, and CCO
- Keynote: US DOJ Civil Section Update
- Keynote: Transparency International's New Pharma Industry Initiative
- Qui Tam Panel
- Keynote: Recent Developments in the Debate over Off-Label Communications and the First Amendment
- Keynote: Update from CMS on Open Payments
- Consolidation, Vertical Integration and Cross-Border Business: Confronting "Next Generation" Compliance Priorities Panel
- Millennials and the Future of Ethics and Compliance Programs: New Technologies and Eternal Issues
|
|
HALF-DAY CLOSED SESSION
|
|
|
- INDUSTRY-ONLY COMPLIANCE BEST PRACTICES THINK TANK
Including an Update from PhRMA
(Industry-only session for pharmaceutical company compliance professionals and in-house counsel only)
|
|
PHARMA CONGRESS IS
|

|
|
|
|
MINI SUMMITS
|
- Mini Summit I: Managing Speaker Program Compliance: Benchmarking and Best Practices
- Mini Summit II: R&D Compliance: Prepare Now for Future Enforcement
- Mini Summit III: Business Development Part 1: Mergers and Acquisitions- Preparing to be Acquired and Pre-Deal Due Diligence
- Mini Summit IV: Evaluating Compliance Program Effectiveness: Board Responsibilities, Board Advisors and Compliance Experts
- Mini Summit V: Payers and Providers Part 1-Evolving Healthcare Systems and Promotional Interactions
- Mini Summit VI: Patient Support Programs Part 1-Getting Closer to the Patient
- Mini Summit VII: Predictive Analytics
- Mini Summit VIII: Coordinating Risk Management across Control Functions- Audit, Compliance, Finance, Legal, Regulatory and Quality - An Opportunity to Leverage Technology
- Mini Summit IX: Managing Multi-National HCP Meetings -Complying with the Codes and Transparency Requirements
- Mini Summit X: Business Development Part 2: Mergers and Acquisitions -Post-Deal Due Diligence and Integration Challenges
- Mini Summit XI: Impact of Public Disclosures on Operations and Processes in Life Science Organizations
- Mini Summit XII: Patient Support Programs Part 2: Privacy and Pharmacovigilance Considerations
- Mini Summit XIII: Innovation in Training and Communications
- Mini Summit XIV: Compliance Considerations when Evaluating Non-Traditional HCP Compensation Arrangements: Royalties, Stock Options and Licensing Agreements
- Mini Summit XV: New Product Launches and the Pre-Launch Period: Strengthening the Partnership with the Business
- Mini Summit XVI: Business Development Part 3: Joint Ventures and Co-Promotion Arrangements
- Mini Summit XVII: Marketing and Medical Affairs: Where is the Line in the Sand?
- Mini Summit XVIII: Structuring Specialty Pharmacy Distribution Arrangements in a Turbulent Regulatory Environment
- Mini Summit XIX: Payers and Providers Part 2: Value Propositions in Contractual Relationships: Real World Evidence, Outcomes Research, and Comparative Effectiveness
|
|
|
|
FEATURED FACULTY
|

Corey R. Amundson, Esq.
First Assistant United States Attorney and Chief, Criminal Division, United States Attorney's Office, Middle District of Louisiana, Baton Rouge, LA |

Jeff Antoon
Director Internal Audit, Johnson & Johnson, New Brunswick, NJ |

Timothy Ayers, JD, MPH
Vice President, Chief Compliance Officer, Horizon Pharma PLC, Former VP, Chief Compliance Officer, Dendreon, Chicago, IL
|

Yogesh Bahl, CPA, MBA
Managing Director, AlixPartners, New York, NY |

Dennis K. Barnes, MBA, JD, CPA
Vice President, Chief Compliance Officer, PAREXEL International, Waltham, MA |

Scott Bass
Global Life Sciences Head, Sidley Austin LLP, Washington, DC
|

Rachel Batykefer, CCEP
Associate Director, Global Compliance, Teva Pharmaceuticals, Frazer, PA |

Gregory Beeman
Lilly USA Ethics and Compliance Officer, Eli Lilly and Company, Indianapolis, IN |

Thomas Beimers, Esq.
Healthcare, Compliance, and Government Investigations Attorney at Faegre Baker Daniels LLP, Minneapolis, MN
|

Andy Bender, MS, MBA
President and Founder, Polaris, New York, NY |

Michael S. Blume, Esq.
Director, Consumer Protection Branch, US Department of Justice, Civil Division, Washington, DC |

Ann S. Brandt, PhD
Partner, HealthCare Appraisers, Inc., Delray Beach, FL
|

Carolyn Bruguera
Vice President Consulting Services, R-Squared, Princeton, NJ |

Eve M. Brunts
Partner, Ropes & Gray LLP, Boston, MA |

Katherine Buckley, MBA
Principal, Pharmaceutical and Life Sciences Advisory Practice, PwC, Philadelphia, PA
|

William Buzzeo, MS
General Manager, US Compliance Business at IMS Health, Plymouth Meeting, PA |

Chris Castro, CCEP
Associate Director, Healthcare & Life Sciences Disputes, Regulatory, Compliance & Investigations, Navigant Consulting, Inc., Phoenix, AZ |

Regina Gore Cavaliere, Esq.
Principal, Advisory, KPMG, Former Vice President & Chief Compliance Officer, Otsuka America Pharmaceutical, Inc., Short Hills, NJ
|

Hwa-Soo Chung, Esq.
Chair, Health Law Group, Kim & Chang Law Firm, Seoul, South Korea |

Kerry Clem
Senior Vice President, Sales, Acorda Therapeutics, Ardsley, NY |

Catherine Clements
Senior Legal Counsel, Express Scripts, Indianapolis, IN
|

Chris Cobourn
Managing Director, Huron Life Sciences, New York, NY |

Kellie Branson Combs, Esq.
Counsel, Ropes and Gray, Washington, DC |

Joseph Coniker, GMP
Principal, National Practice Leader, Business Analytics, Grant Thornton, Philadelphia, PA
|

Brian J. Conner
Senior Director, Asst. Corporate Compliance Officer, Shire, Philadelphia, PA |

John Conrad
Principal, Deloitte, Philadelphia, PA |

Thomas E. Costa, Esq.
Compliance Consultant, Former Vice President, US Pharmaceuticals Compliance, Bristol-Myers Squibb, Yardley, PA
|

Traci Coughlan, Esq.
Principal, Advisory Services, The Red Flag Group, Berlin, MD |

Kris Curry, MBA
Principal, Fraud Investigation and Dispute Services, Ernst & Young LLP, Former Vice President, Health Care Compliance, Pharmaceuticals Group, Johnson & Johnson, Philadelphia, PA |

Paul Curtin, JD, CFE
Compliance Officer - Global Research and Development, Actavis, Former Head of Compliance - Ex-US, Forest Laboratories, New York, NY
|

BJ D'Avella, MBA
Senior Director, Huron Life Sciences, New York, NY |

Sujata Dayal, Esq.
Vice President Health Care Compliance and Privacy, Pharmaceuticals, Johnson & Johnson; Former Global Chief Compliance Officer and Corporate Vice President, Biomet, Inc., Former Member PCF Executive Committee, Chicago, IL |

Mark DeWyngaert, MBA, PhD
Managing Director, Huron Life Sciences, New York, NY
|

Justin Dillon
Senior Director - Compliance, Auditing & Monitoring, Incyte, Wilmington, DE |

Daniel L. Dovdavany, Esq.
Assistant General Counsel North America Litigation & Investigations, Sanofi US, Bridgewater, NJ |

Corey Dunbar, CFE
Senior Manager, Fraud Investigations & Dispute Services, Ernst & Young LLP, New York, NY
|

Nathaniel B. Edmonds, Esq.
Partner, Paul Hastings, Former Assistant Chief, Foreign Corrupt Practices Act Unit, Fraud Section, Criminal Division, US Department of Justice, Washington, DC |

Marc Eigner, MBA, MS
Senior Partner and Co-Founder, Polaris, New York, NY |

Jill Fallows-Macaluso
Vice President, Chief compliance Officer, Novo Nordisk Inc., Plainsboro, NJ
|

Margaret K. Feltz, MA, JD
Executive Director, Corporate Compliance, Purdue Pharma LP, Former Member, PCF Executive Committee, Stamford, CT |

Christopher Fletchall, MBA
Senior Director, Ethics and Compliance, Eli Lilly and Company, Indianapolis, IN |

Andrew Foose, JD
Vice President, NAVEX Globalís Advisory Services, Washington, DC
|

Jeff Francer, Esq.
Vice President and Senior Counsel, PhRMA, Former Associate Chief Counsel, Food and Drug Administration, Washington, DC |

Henrique Frizzo
Partner, Life Sciences and Public Law, Trench, Rossi e Watanabe, A Baker McKenzie Affiliate, São Paulo, Brazil |

Ronni E. Fuchs, Esq.
Partner, Pepper Hamilton LLP, Princeton, NJ
|

James Gibney
Senior Director of Compliance, Regeneron Pharmaceuticals; Former Director, Worldwide Programs and US Investigations - Corporate Compliance, Pfizer, Tarrytown, NY |

Virginia "Ginny" A. Gibson, Esq.
Partner, Hogan Lovells LLP, Former Executive Assistant U.S. Attorney, Eastern District of Pennsylvania, Philadelphia, PA |

Thomas M. Glavin
Vice President, Head of Compliance - US, Shire, Wayne, PA
|

Edward Glynn, MBA
Principal, Ernst & Young LLP, New York, NY |

William Greenrose, MA, MBA
Director, Advisory - Governance Regulatory and Risk Strategies Practice, Deloitte & Touche, LLC, Boston, MA |

Tali Guy, Esq.
Senior Director, Global R&D Compliance Officer, Teva Pharmaceutical Industries Ltd., Petah Tikva, Israel
|

Kathleen Hamill
Assistant General Counsel, Johnson & Johnson, Philadelphia, PA |

Samantha Hand
Global Training Manager, Johnson & Johnson, Titusville, NJ |

Jeffrey L. Handwerker, Esq.
Partner, Arnold & Porter, Washington, DC
|

Samir Hans, MS
Principal at Deloitte, Washington, DC |

Wendy Heckelman, PhD
President, WLH Consulting, Inc., Fort Lauderdale, FL |

Saul Helman, MD, MBA
Managing Director and Life Sciences Practice Leader, Navigant Consulting, Inc., Indianapolis, IN
|

William I. Intner, Esq.
Partner, Hogan Lovells LLP, Baltimore, MD |

Mark A. Jensen, Esq.
Partner, Special Matters and Government Investigations Team, King & Spalding, Washington, DC |

Michael Joachim, Esq.
Head of Global Corporate Compliance, Genzyme, Cambridge, MA
|

Paul E. Kalb, JD, MD
Partner and Global Coordinator, Life Sciences Practice, Sidley Austin LLP, Washington, DC |

Gary Keilty
Managing Director, FTI, Washington, DC |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC
|

Daniel A. Kracov, Esq.
Partner and Head, FDA and Healthcare Practice, Arnold & Porter, Washington, DC |

Holly Kramen
Vice President, Global Compliance Officer, Circassia Pharmaceuticals Inc., Chicago, IL |

Michael S. Labson
Partner, Covington & Burling LLP, Washington, DC
|

Joanne Lahner, Esq.
Vice President, GHH Compliance at Merck, North Wales, PA |

Stephen M. Lawniczak, Esq.
Senior Counsel, AbbVie, Chicago, IL |

Terri Ledva, MS
Senior Manager Compliance, Iroko Pharmaceuticals LLC, Philadelphia, PA
|

Natasha Leskovsek, RN, MPM, MBA, JD
Health Care & Life Sciences Regulatory Practice, Cooley, LLP, Washington, DC |

John "Jack" S. Linehan, Esq.
Associate, Epstein Becker Green, Former Special Assistant US Attorney, US Attorney's Office for the District of Maryland, Washington, DC |

Mark Linver, MS
Managing Director, Huron Life Sciences, New York, NY
|

Kari Loeser, Esq.
Senior Counsel, Jazz Pharmaceuticals, Palo Alto, CA |

Christine Longawa, CPA, CFE
Associate Director, Healthcare & Life Sciences Disputes, Regulatory, Compliance and Investigations, Navigant Consulting, Inc., Chicago, IL |

Phillip Lo Scalzo, Esq.
Senior Vice President, Chief Compliance Officer, BioMarin Pharmaceutical Inc., Novato, CA
|

Michael K. Loucks, Esq.
Partner, Skadden Arps LLP; Former Acting United States Attorney, US Attorney's Office for the District of Massachusetts, Washington, DC |

Seth H. Lundy, Esq.
Partner, King & Spalding LLP, Washington, DC |

Maureen McGirr, Esq.
Vice President, Office of Ethics, Global Compliance Organization, Merck & Co., Inc., Kenilworth, NJ
|

Gary Mendelsohn
Assistant Director, Corporate Compliance For Oncology, Astellas, Greer, SC |

Megan Mikkelsen, CIPP/US, CHPS
Director, US Chief Privacy Officer, Teva Pharmaceuticals, Kansas City, MO |

James Moran, JD, CPA
Managing Director, PwC, St. Louis, MO
|

Ned Mumtaz, MBA, MIS
Practice Leader and Associate Partner, Streebo Inc., Princeton, NJ |

Stuart A. Neiberg, MAcc, CPA, CFA
Director, HealthCare Appraisers, Delray Beach, FL |

George K. Ng, Esq.
Executive Vice President & Chief Legal Officer, Sorrento Therapeutics, Inc., San Diego, CA
|

Jennifer Neuenhoff, CPA
Associate Director, Compliance Auditing and Monitoring, Allergan PLC, Parsippany, NJ |

MaryAnn Northrup, CFE, CHRC
Senior Director, FTI Consulting, Indianapolis, IN |

Michael O'Connor, MS
Senior Director, Compliance and Ethics, Alexion Pharmaceuticals, Inc., Cheshire, CT
|

Neely O'Donnell
Senior Manager, Corporate Compliance, Biogen, Cambridge, MA |

John Patrick Oroho, Esq.
Executive Vice President & Chief Strategy Officer, Porzio Life Sciences, LLC; Principal, Porzio Bromberg & Newman, PC, Morristown, NJ |

Gus Papandrikos, MBA
Global Head of Compliance Operations, Shire; Former Senior Director, Compliance, Daiichi Sankyo, Parsippany, NJ
|

Amy Pawloski, CPA, CCEP
Head of US Compliance & Ethics Monitoring and Data Analytics, Bristol-Myers Squibb, Plainsboro, NJ |

Kathleen Peterson, Esq.
Special Counsel, Cooley LLP, Washington, DC |

Kelly N. "Nikki" Reeves, MPA, JD
Partner, King & Spalding LLP, Legal Counsel, Ad Hoc Sunshine and State Law Compliance Group, Washington, DC
|

Terra L. Reynolds, Esq.
Of Counsel, Litigation Department, Paul Hastings LLP, Assistant U.S. Attorney, Northern District of Illinois, Chicago, IL |

Vivian Robinson, Esq.
Queen's Counsel, Partner, McGuire Woods, Former General Counsel of the UK Serious Fraud Office, Former Head, QEB Hollis Whiteman Chambers, Recorder of the Crown Court and Treasurer of Inner Temple, London, UK |

Janet L. "Lucy" Rose, MBA
President, Lucy Rose and Associates, LLC, Former Director, DDMAC, FDA, Washington, DC
|

Lee H. Rosebush, JD, PharmD, MS, MBA
Pharmacy Team Leader and FDA Partner, Baker &Hostetler LLP, Washington, DC |

Lauren K. Roth, Esq.
Assistant General Counsel, PhRMA, Washington, DC |

Barbara Rowland, Esq.
Principal, Internal Investigations and White Collar Defense Group, Post & Schell, PC, Washington, DC
|

Matthew Ruble, MS
Senior Manager, Grant Thornton, Philadelphia, PA |

Ben Rymzo
Managing Director, Huron Life Sciences, Boston, MA |

Elizabeth Schwartz, MBA, CCEP
Director, US External Reporting and Transparency, Global Operations, Johnson & Johnson, New York, NY
|

John D. Shakow, Esq.
Partner, FDA& Life Sciences Practice, King & Spalding, Washington, DC |

Paul Silver
Practice Leader and Managing Director, Huron Life Sciences, Atlanta, GA |

Scot Steinheiser, CHC, CCEP
Managing Partner and Founder, Commercial Compliance Consulting, SCS Compliance Group LLC, Mundelein, IL
|
David Stollman
Associate Director, Healthcare & Life Sciences Disputes, Regulatory, Compliance and Investigations, Navigant Consulting, Inc., Philadelphia, PA |

Terrell Sweat, MBA
Compliance Director, Actelion Pharmaceuticals, South San Francisco, CA |

Aditi Taylor, MBA
Principal, Deloitte Advisory, Deloitte and Touche LLP, Boston, MA
|

Yuet Ming Tham, Esq.
Partner, Sidley Austin LLP, Former, Asia-Pacific Regional Compliance Director, Pfizer, Former Deputy Public Prosecutor, Singapore, Hong Kong |

Fernando Villegas, MS
Director, Oncology Marketing, Jazz Pharmaceuticals, Palo Alto, CA |

Seth B. Whitelaw, JD, LLM, SJD
President and Chief Executive Officer, Whitelaw Compliance Group, LLC; Editor-in-Chief, Life Science Compliance Update, West Chester, PA
|

Jonathan Wilkenfeld, MBA
President, Potomac River Partners, Vienna, VA |

Susan Williamson
Vice President and US Compliance Officer, Endo Pharmaceuticals, Philadelphia, PA |

Stephanie Wisdo, Esq.
Corporate Attorney, Otsuka America Pharmaceutical, Inc., Princeton, NJ
|

Raymond S. Wysmierski, MBA, MJP
Associate Director, Compliance, Avanir Pharmaceuticals, Inc, Aliso Viejo, CA |

Joseph Zimmerman
Former Senior Vice President and Chief Compliance Officer, Actavis plc; Former Global Chief Compliance Officer, Forest Laboratories, New York, NY
|
|
|
|
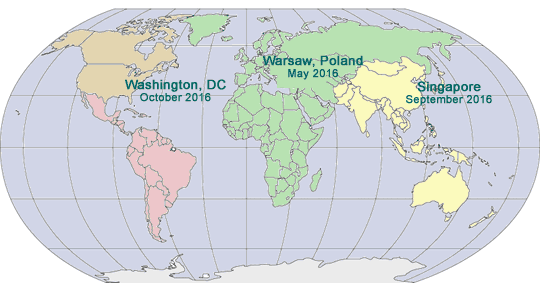
2016 GLOBAL PHARMA COMPLIANCE CONGRESSES
|
|
TENTH INTERNATIONAL PHARMACEUTICAL
COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Cosponsored by Pharmaceutical Compliance Forum (PCF)
May 10 - 12, 2016
Hilton Warsaw Hotel and Convention Centre
Warsaw, Poland
www.InternationalPharmaCongress.com
|
|
SIXTH ASIA PACIFIC
PHARMACEUTICAL
COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by International Society of Healthcare Ethics and Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
September 2016
Singapore
www.AsianPharmaCongress.com
|
|
SEVENTEENTH PHARMACEUTICAL REGULATORY AND COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Transformational Learning - Effective Knowledge Exchange
Sponsored by Pharmaceutical Compliance Forum
Media Partners: Harvard Health Policy Review, Health Affairs and Life Sciences Compliance Update
October 19 - 21, 2016
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
|
|
|
OTHER GLOBAL PHARMA CONGRESS CITIES
|

2007 - Brussels

2008 - Paris

2009 - Rome

2010 - Berlin

2011 - Istanbul
|

2011 - Singapore

2012 - Budapest

2012 - Shanghai

2012 - São Paulo

2013 - Madrid
|

2013 - Kuala Lumpur

2014 - Dubai

2014 - Mexico City

2014 - Shanghai

2015 - Manila
|
|
|
|

|
BROCHURE NOW AVAILABLE
|
Click here to download.
|
|
|
|
SPONSORED BY:
|

The Pharmaceutical Compliance Forum (PCF) is a coalition of senior compliance professionals and legal counsel from more than 50 of the largest research-based pharmaceutical manufacturers. The PCF was founded in early-1999 by compliance professionals from the pharmaceutical industry to promote effective corporate compliance programs. The members meet twice a year, for two days, focusing on open and informal sharing of compliance information, best practices, and current developments in the field, and sponsors a two-day international compliance congress in the Spring and a three-day US compliance congress each Fall.
|
|
CO CHAIRS
|

Matthew D'Ambrosio, MBA, JD
Senior Vice President, Chief Compliance and Ethics Officer, Sunovion Pharmaceuticals Inc., Marlborough, MA
|

Gary DelVecchio
Executive Director, US Pharmaceutical Compliance and Ethics, Bristol-Myers Squibb Company, Plainsboro, NJ
|

James Gibney
Senior Director of Compliance, Regeneron Pharmaceuticals; Former Director, Worldwide Programs and US Investigations - Corporate Compliance, Pfizer, Tarrytown, NY
|

Elizabeth Jobes, Esq.
Legal Counsel and Head of Corporate Compliance, Spark Therapeutics, Philadelphia, PA
|

Glenna Shen, JD, MBT
Executive Director, Worldwide Compliance and Business Ethics, Amgen Inc., Thousand Oaks, CA
|
|
|
INVITATION TO ATTEND THE PCF PHARMA CONGRESS
|
|
As the new Vice President, Pharma and Medical Device Content, Global Health Care - Life Sciences, LLC, it is my pleasure to invite you to attend the 2015 Sixteenth Annual Pharmaceutical Regulatory and Compliance Congress and Best Practices Forum sponsored by the PCF. This year's Congress will feature presentations by leading government regulators, company compliance professionals, in-house counsel, prominent industry consultants and legal counsel. Please plan to join me and the Planning Committee for three stimulating days of continuing education, networking and best practice sharing.
|

Kelly B. Freeman, PhD
Vice President, Pharma and Medical Device Content, Global Health Care - Life Sciences, LLC, Former Senior Advisor, Ethics and Compliance, Eli Lilly and Company, Former Member, PCF Executive Committee, Indianapolis, IN
|
|
|
MEDIA PARTNERS:
|



|
|
GRANTORS:
|
|
GOLD
|

|
|
SILVER
|




|
|
BRONZE
|














|
|
CONTINUING EDUCATION CREDITS:
|
Accounting Professionals: Approved for up to 17.5 NASBE CPE credits.
Compliance Professionals: The Congress has been approved for 22.5 Compliance Certification Board (CCB) Credits.
Attorneys: Approved to offer a maximum of 11.5 Hours of Pennsylvania CLE Credits.
Attorneys: The Congress is currently pending approval to offer California MCLE Credit.
Click here for more information.
|
|
2015 PCF PHARMA CONGRESS PLANNING COMMITTEE
|
Matthew D'Ambrosio, JD, MBA
Senior Vice President, Chief Compliance and Ethics Officer, Sunovion Pharmaceuticals, Inc., Marlborough, MA (Co-chair)
Gary DelVecchio
Executive Director, US Pharmaceutical Compliance and Ethics, Bristol-Myers Squibb, Plainsboro, NJ (Co-chair)
Elizabeth V. Jobes, Esq.
Head of Corporate Compliance and Legal Counsel, Spark Therapeutics, Philadelphia, PA (Co-chair)
Timothy Ayers, JD, MPH
Vice President, Chief Compliance Officer, Horizon Pharma plc, Chicago, IL
Scott Bass, Esq.
Partner and Head, Global Life Sciences Team, Sidley Austin LLP, Washington, DC
John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP, Washington, DC
Yogesh Bahl, MBA
Managing Director, AlixPartners, New York, NY
Andy Bender, MS, MBA
President and Founder, Polaris, New York, NY
Mary Bradley, PharmD
Healthcare Compliance Officer, Johnson & Johnson, Philadelphia, PA
Eve Costopoulos, Esq.
Former Vice President and Chief Ethics and Compliance Officer, Eisai Inc., Woodcliff Lake, NJ
Kris Curry, MBA
Principal, Fraud Investigation and Dispute Services, Ernst & Young LLP, Philadelphia, PA
Clive Davis, MA, JD
Vice President, Chief Compliance Officer , UCB Pharma, Atlanta, GA
Michael B. Dusseau
Vice President, Compliance Operations, Actavis, Inc., Parsippany, NJ
Margaret K. Feltz, MA, JD
Executive Director, Corporate Compliance, Purdue Pharma LP, Stamford, CT
Alison Fethke, Esq.
Counsel, Health Care Group, Ropes & Gray, Chicago, IL
Jeffrey Fleming, Esq.
Vice President Compliance North America and US Compliance Officer, AstraZeneca Pharmaceuticals LP, Wilmington, DE
Thomas M. Glavin
Vice President, Head of Compliance - US, Shire, Wayne, PA
Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings, New York, NY
Virginia "Ginny" A. Gibson, Esq.
Partner, Hogan Lovells LLP, Philadelphia, PA
Wendy C. Goldstein, Esq.
Partner, Health Care & Life Sciences Regulatory Practice, Cooley, LLP, New York, NY
Steven Guymon
Senior Compliance Advisor, Eli Lilly and Company, Indianapolis, IN
Alessandra Hawthorne, Esq.
Vice President, Chief Ethics and Compliance Officer, Boehringer-Ingelheim Pharmaceuticals, Ridgefield, CT
Wendy Heckelman, PhD
President and Founder, WLH Consulting, Inc., Fort Lauderdale, FL
Erinn Hutchinson
Partner, Advisory Services, PricewaterhouseCoopers, Philadelphia, PA
Margaret Jackson
National Industry Marketing Director, Grant Thornton LLP, Raleigh, NC
Michael Joachim, Esq.
Head of Global Corporate Compliance, Genzyme, a Sanofi Company, Cambridge, MA
Jeffrey S. Klimaski, MBA, CPA
Vice President, Global Ethics and Compliance Officer, BTG International Inc., Philadelphia, PA
Daniel A. Kracov, Esq.
Partner and Head, FDA and Healthcare Practice, Arnold & Porter, Washington, DC
Terri Ledva, MS
Ethics & Compliance Leader, Iroko Pharmaceuticals, Philadelphia, PA
Beth Levine, Esq.
Vice President, Associate General Counsel, Chief Compliance Officer, Regeneron, New York, NY
Christine Longawa, MA, CPA, CFE
Life Sciences Disputes, Compliance & Investigations Associate Director, Navigant Consulting, Chicago, IL
Maureen McGirr, Esq.
Vice President Office of Ethics, Global Compliance Organization , Merck, Philadelphia, PA
Maxine Nogard
Compliance Director, Biogen Idec, Weston, MA
John Patrick Oroho, Esq.
Executive Vice President and Chief Strategy Officer, Porzio Life Sciences, LLC, Principal, Porzio, Bromberg & Newman PC, Morristown, NJ
Jeremy Perisho
Partner and US FAS Leader, Life Sciences & Health Care, Deloitte Financial Advisory Services LLP, Boston, MA
Lori Queisser
Senior Vice President and Global Chief Compliance Officer, Teva Pharmaceuticals, Horsham, PA
Kelly N. "Nikki" Reeves, MPA, JD
Partner, King & Spalding LLP, Washington, DC
Jeffrey Rosenbaum, MBA
Vice President, Chief Compliance Officer, Vertex Pharmaceuticals, Cambridge, MA
Michael Shaw, Esq.
Vice President and Compliance Officer, North America Pharmaceuticals & Global Franchises, GlaxoSmithKline, Philadelphia, PA
Glenna Shen, Esq.
Head of Compliance, Onyx Pharmaceuticals, South San Francisco, CA
Paul Silver
Practice Leader and Managing Director, Huron Life Sciences, Atlanta, GA
David Vance, Esq.
Senior Director Business Conduct, Noven Pharmaceuticals, Inc., Miami, FL
Seth B. Whitelaw, JD, PhD
President & Chief Executive Officer, Whitelaw Compliance Group, LLC, West Chester, PA
Raymond Wysmierski, MBA, MJP
Associate Director, Compliance, Avanir Pharmaceuticals, Aliso Viejo, CA
|
|
FOLLOW PHARMA CONGRESS ON
|


|
|
PARTICIPATION OPTIONS
|
|
|
|
TRADITIONAL ONSITE ATTENDANCE
|
Simply register, travel to the conference city and attend in person.
Pros: subject matter immersion; professional networking opportunities; faculty interaction

|
|
LIVE AND ARCHIVED WEBCAST PARTICIPATION
|
Watch the conference in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office


|
WEBCAST INTERFACE SAMPLE
|
Click here for a sample stream
|
|

 This site complies with the HONcode standard for trustworthy health information: This site complies with the HONcode standard for trustworthy health information:
verify here.


|
THE 2014 FIFTEENTH ANNUAL PHARMA CONGRESS CONTENT IS NOW AVAILABLE IN VARIOUS POST CONFERENCE FORMATS
|
|
The 2014 Fifteenth Annual Pharma Congress conference content is now available in a variety of formats.
You may purchase the Congress streaming content in the following formats: Flash Drive or online archive (6 months). You may also purchase individual presentations in an online archive (6 months) format.
YOU CAN PURCHASE JUST THE PHARMA CONGRESS CONTENT AS FOLLOWS:
|

Online Archive of 2014 Fifteenth Annual Pharma Congress Presentations today!
$595
Order Now

Flash Drive of 2014 Fifteenth Annual Pharma Congress Presentations today!
$595
Order Now
|
|
FINALLY YOU MAY PURCHASE PHARMA CONGRESS INDIVIDUAL PRESENTATIONS:
Click here to purchase individual presentations for $59.95 in online archive format (6 months of access - 24/7).
|


|
|

|

