
|

Sponsored by
The Pharmaceutical
Compliance Forum
|
SEVENTEENTH ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
Transformational Learning - Effective Knowledge Exchange
October 19 - 21, 2016
Mandarin Oriental
Washington, DC
|
|
KEYNOTE SPEAKERS
|

Thomas W. Abrams, RPh, MBA
Director, Office of Prescription Drug Promotion, Food and Drug Administration, Silver Spring, MD |

Joseph Beemsterboer, JD
Deputy Chief, Health Care Fraud Unit, Criminal Division, U.S. Department of Justice, Washington, DC |

Sophie Peresson, LLM, MA
Director, Pharmaceuticals & Healthcare Programme, Transparency International UK, London, UK
|

Tracy L. Price, JD
Assistant Director, FCPA Unit, Division of Enforcement, U.S. Securities and Exchange Commission, Washington, DC |

Mary E. Riordan, Esq.
Senior Counsel, Office of Counsel to the Inspector General, Office of Inspector General, US Department of Health and Human Services, Washington, DC
|
|
|
|
CHIEF COMPLIANCE OFFICER ROUNDTABLE
|

Jill Fallows Macaluso, Esq.
Chief Compliance Officer and Vice President, Novo Nordisk Inc., Princeton, NJ |

Jeffrey Fleming, JD
Vice President and Chief Compliance Officer, Vertex Pharmaceuticals, Boston, MA |

Jonathon Kellerman
Executive Vice President, Global Chief Compliance Officer, Allergan PLC, Parsippany, NJ
|

Lori Queisser
Senior Vice President and Global Chief Compliance Officer, Teva Pharmaceuticals, Horsham, PA |

Michael L. Shaw, Esq.
Vice President and Compliance Officer, GlaxoSmithKline-NA Pharmaceuticals, Former Member, PCF Executive Committee, Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services, Philadelphia, PA
|

Caroline West
Global Chief Compliance Officer, Olympus Corporation, Former Senior Vice President, Chief Compliance and Risk Officer, Shire, Philadelphia, PA, USA |

Paul Silver
Practice Leader and Managing Director, Huron Consulting Group, Atlanta, GA (Moderator)
|
|
|
|
FEATURING PRECONFERENCE SESSIONS
|
|
|
- Managed Markets 101: Overview of the US Payment Systems for Pharmaceuticals
- Being Prepared for External Investigations, Subpoenas, and OIG Monitor Interactions
- Advanced Global Compliance Issues
|
|
AN INVITATION-ONLY CCO MEETING
|
|
PLENARY SESSIONS
|
|
|
- Overview of the US Pharmaceutical Marketplace and Politics
- Chief Compliance Officer Roundtable
- Keynote: Annual OIG Update
- Keynote: Transparency International
- FCPA Enforcement Panel
- Annual AUSA Roundtable
- Annual FDA-OPDP Update
- Behind the Bribe: Multiple Real-World Perspectives
- Truthful and Non-Misleading Communications and 1st Amendment Cases
- Evolution of Compliance Programs into Systems Supporting Business Integrity
- Recent Developments in Executive Liability: Vascular Solutions, Warner-Chilcott, and Acclarent
|
|
PHARMA CONGRESS IS
|

|
|
|
|
MINI SUMMITS
|
- Compliance Considerations for the Managed Markets Business
- R&D Compliance Best Practices
- New Ethics-Based Approaches to Policies
- Organizational for Global Transparency
- Enhancing Third Party Oversight and Due Diligence
- Leveraging Analytics for Monitoring
- Leveraging Publically Available Data
- Reimbursement Support, Patient Assistance, Coupons and Charitable Foundations
- Medical Affairs Compliance
- Compliance 2.0: Effective Compliance Across Business Functions
- What's New for Training Programs
- Third Parties: Risk Assessments, FMV, Monitoring and Auditing
- Managed Markets Risk Assessment and Monitoring
- Beyond Transparency: HCP Interaction Risk Management
- Hubs and Specialty Pharmacy Arrangements: Structuring Service Agreements
- Compliance Issues for Medical Devices: Working through Distributors
- The New Marketplace: Value-Based Contracting
- Evolution of Risk Assessment and Management Programs
- Current Initiatives from the Trade Associations
|
|
AND AN INDUSTRY-ONLY COMPLIANCE BEST PRACTICES THINK TANK
|
|
|
|
ANNUAL AUSA ROUNDTABLE
|

Kenneth Abell, Esq.
Chief, Civil Health Care Fraud, United States Attorney's Office, Eastern District of New York, US Department of Justice, New York, NY |

Jacob Elberg, Esq.
Chief, Health Care and Government Fraud Unit, US Attorney's Office, District of New Jersey, US Department of Justice, Newark, NJ
|

Gregg Shapiro, Esq.
Assistant US Attorney, US Attorney's Office, District of Massachusetts, US Department of Justice, Boston, MA |

John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP; Former Special Counsel for Healthcare Fraud and Chief Privacy Officer, US Department of Justice, Washington, DC
|
|
|
FEATURING BEHIND THE BRIBE: MULTIPLE REAL-WORLD
PERSPECTIVES ON HOW FOREIGN BRIBERY OCCURS, IS
INVESTIGATED AND COULD BE PREVENTED BY
|

Richard Bistrong
Chief Executive Officer, Front-Line Anti-Bribery LLC, Contributing Editor, The FCPA Blog, New York, NY |

George "Ren" McEachern, CFE, CAMS
Supervisory Special Agent, Federal Bureau of Investigation, Washington, DC |

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings, Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY (Moderator)
|
|
|
PERSPECTIVES FROM THE DEFENSE:
RECENT PROSECUTIONS OF CORPORATE EXECUTIVES BY
|

William Hassler
Partner, Steptoe & Johnson LLP, Defense Counsel for William Facteau, Former CEO, Acclarent, Inc., Washington, DC |

John W. Lundquist, Esq.
Shareholder, Fredrikson and Byron, P.A., Defense Counsel for Howard Root, CEO, Vascular Solutions, Inc., Minneapolis, MN
|

Joseph F. Savage, Jr., Esq.
Partner, Goodwin Procter LLP, Defense Counsel for W. Carl Reichel, Former President, Warner Chilcott, Boston, MA |

Daniel A. Kracov, Esq.
Partner and Head, FDA and Healthcare Practice, Arnold & Porter, Washington, DC (Moderator)
|
|
|
TRUTHFUL AND NON-MISLEADING COMMUNICATIONS
AND RECENT FIRST AMENDMENT CASES
|

Floyd Abrams, JD
Partner, Cahill Gordon & Reindel LLP, Represented Amarin Corporation in Amarin Pharma, Inc., et al. v. FDA et al. No. 15-3588 (S.D.N.Y. May 7, 2015), New York, NY |

Kellie B. Combs, Esq.
Counsel, Ropes & Gray, Co-counsel to Medical Information Working Group, Represented Pacira Pharmaceuticals in Pacira Pharmaceuticals, Inc. v. FDA, 15-cf-07055 (SDNY Sept. 8, 2015), Washington, DC |

Lisa Dwyer, Esq.
Partner, King & Spalding, Former Senior Policy Advisor, Office of Policy, Food and Drug Administration, Former Deputy Chief of Staff to the Commissioner, Food and Drug Administration, Washington, DC
|

Joshua M. Sharfstein, MD
Associate Dean for Public Health Practice and Training, Johns Hopkins Bloomberg School of Public Health; Former Principal Deputy Commissioner, U.S. Food and Drug Administration; Former Health Policy Advisor for Congressman Henry A. Waxman, Baltimore, MD |

Coleen Klasmeier, JD
Partner, Sidley Austin LLP, Co-Counsel for Medical Information Working Group, Washington, DC (Moderator)
|
|
|
|
FEATURED FACULTY
|

Michael Ace, MS
Advisor, Ethics and Compliance, Global Policies and Standards, Eli Lilly and Company, Indianapolis, IN |

Sergio Alegre, JD
Vice President, Global Compliance, Osmotica Pharmaceutical LLC; Former Vice President and Global Chief Compliance Officer, Vertical Pharmaceuticals, Bridgewater, NJ |

Anthony Alvizu, CPA, EnCE, CFE
Managing Director, Global Risk and Investigations Practice, FTI Consulting, Chicago, IL
|

Jose M. Ayala, MBA
Program Director, Global Channel Compliance, Medtronic, Minneapolis, MN |

Yogesh Bahl, CPA, MBA
Managing Director, Alix Partners, New York, NY |

Eric Baim, JD, MA
Vice President, Head of Compliance US, Shire, Boston, MA
|

Ann Beasley, Esq.
Director, Navigant, Former Senior Vice President, Chief Compliance Officer, Biogen, Boston, MA |

Gregory Beeman
Lilly USA Ethics and Compliance Officer, Eli Lilly and Company, Indianapolis, IN |

Thomas W. Beimers, Esq.
Partner, Hogan Lovells; Former Senior Counsel for Administrative and Civil Remedies, Office of the Inspector General, Department of Health and Human Services, Minneapolis, MN
|

Andy Bender, MS, MBA
President and Founder, Polaris, New York, NY |

Ela Bochenek, JD
Former Vice President, Global Compliance, Insmed Incorporated, Bridgewater, NJ |

Marc Alain Bohn
Counsel, Miller & Chevalier Chartered, Washington, DC
|

Kara Bonitatibus, JD
Assistant General Counsel, Compliance Lead for Compliance Policy & Strategic Operations, Pfizer, Inc., New York, NY |

Anthony Brennan, CPA, CFE
Senior Manager, Fraud Investigation & Dispute Services, EY, Former Senior Director, Governance, Metrics and Reporting, Health Care Compliance, Johnson & Johnson, Iselin, NJ |

Victoria Browning, CCEP
Compliance Officer at KARL STORZ North America, El Segundo, CA
|

Katherine Buckley, MBA
Principal, PwC Risk Consulting, Philadelphia, PA |

Valerie Burkholder
Global Process Owner - FMV, Eli Lilly and Company, Indianapolis, IN |

Jeffrey Campbell, JD
President and Chief Executive Officer, Porzio Life Sciences, Porzio Life Sciences, LLC, Morristown, NJ
|

Ellen Carman
Manager, Business Advisory Services, Life Sciences Sector, Grant Thornton LLP, Philadelphia, PA |

Michael Casey
Counsel, Ropes and Gray LLP, Washington, DC |

Michael Clarke, JD
Vice President, Corporate Compliance, Indivior Inc., Richmond, VA
|

Chris Cobourn
Managing Director, Huron Consulting Group, New York, NY |

Jonathan Connell, JD, MBA
Senior Counsel, Bristol-Myers Squibb, Plainsboro, NJ |

Brian J. Conner
Director, Huron Consulting Group; Former Senior Director, Assistant, Compliance Officer, Global Compliance, Shire Pharmaceuticals, Atlanta, GA
|
Colleen A. Conry, Esq.
Partner, Ropes and Gray LLP; Former Senior Litigation Counsel, Fraud Section, Criminal Division, United States Department of Justice, Washington, DC |

Thomas E. Costa, JD
Compliance Consultant; Former Vice President U.S. Compliance and Ethics, Bristol-Myers Squibb, Princeton, NJ |

Traci Coughlan, Esq.
Principal, Advisory Services, The Red Flag Group, Berlin, MD
|

Clarissa Crain
Senior Manager, Life Sciences & Health Care, Deloitte & Touche LLP, Philadelphia, PA |

Kris Curry, MBA
Principal, Fraud Investigation and Dispute Services, Ernst & Young LLP, Former Vice President, Health Care Compliance, Pharmaceuticals Group, Johnson & Johnson, Philadelphia, PA |

Michaeline Daboul
President and Chief Executive Officer, MMIS, Inc., MediSpend, Portsmouth, NH
|

Meenakshi Datta, JD
Partner, Sidley Austin LLP, Chicago, IL |

BJ D'Avella, MBA
Senior Director, Huron Consulting Group, New York, NY |

Neil DeHenes
Senior Manager, Deloitte & Touche LLP, Tampa, FL
|

John DeMarrais, MBA
Managing Director, Deloitte & Touche LLP, Parsippany, NJ |

Mark DeWyngaert, MBA, PhD
Managing Director, Huron Life Sciences, New York, NY |

Tom DiLenge, JD
President, Policy, Advocacy, and Law Division, Biotechnology Innovation Organization, Former Chief Counsel and Policy Director, House Homeland Security Committee, Former Senior Counsel, House Energy and Commerce Committee, Washington, DC
|

Daniel L. Dovdavany
Associate General Counsel, North America Litigation and Investigations, Sanofi US, Bridgewater, NJ |

Michael B. Dusseau
Vice President, Compliance Operations, Allergan plc, Parsippany, NJ |

Nathaniel B. Edmonds, JD
Partner, Paul Hastings; Former Assistant Chief, Foreign Corrupt Practices Act Unit, Fraud Section, Criminal Division, US Department of Justice, Washington, DC
|

Kevin L. Espinoza, MBA
Global Vice President, Ethics & Compliance, BTG International, Durham, NC |

Margaret Feltz, JD, MA
Executive Director, Acting Chief Compliance Officer, Ethics & Compliance, Purdue Pharma L.P., Stamford, CT |

George Fife
Partner, Fraud Investigation & Dispute Services, EY, Former Executive Director, Compliance & Ethics, Bristol-Myers Squibb, Paris, France
|

Sarah Anne Franklin, Esq.
Partner, Covington & Burling LLP, Washington, DC |

Jonathan Glazier, JD, MBA
Senior Legal Counsel, Legal Compliance, Philips Electronics North America, Andover, MA |

Edward Glynn, MBA
Principal, Ernst & Young LLP, New York, NY
|

Gejaa Gobena, Esq.
Partner, Hogan Lovells, Former Deputy Chief, Criminal Division, Fraud Section, United States Department of Justice, Washington, DC |

Anthony Greco, MBA
Director, Pharmaceutical & Life Sciences Advisory Services, PwC, Philadelphia, PA |

Thomas A. Gregory, CPA, CFA
Partner, Fraud Investigation and Dispute Services, Ernst & Young LLP, Atlanta, GA
|

Jeffrey L. Handwerker, JD
Partner and Head, FDA and Healthcare Practice, Arnold & Porter, Washington, DC |

Stacy Hornaday
Senior Manager, Deloitte & Touche LLP, Chicago, IL |

William Hrubes, MHA
Vice President, Chief Compliance Officer, ACell, Inc., Columbia, MD
|

Pamela Hrubey, CCEP
Managing Director, Crowe Horwath LLP, Former Director, Enterprise Risk Management, Eli Lilly and Company, Indianapolis, IN |

Duane Hughes
Consultant, Ethics and Compliance, Eli Lilly and Company, Oak Park, IL |

Michael Joachim, Esq.
Global Business Partner, Ethics and Business Integrity, Sanofi Genzyme, Cambridge, MA
|

Gary Keilty
Managing Director, FTI, Washington, DC |

Kristin Graham Koehler, JD
Partner, Sidley Austin LLP, Washington, DC |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC
|

Terri Ledva, MS
Former Senior Manager Compliance, Iroko Pharmaceuticals LLC, Philadelphia, PA |

Lisa LaMond, MBA
Executive Director, US Market Strategy & Planning, Merck & Co Inc., Kenilworth, NJ |

Susan Lee, JD
Partner, Hogan Lovells LLP, Washington, DC
|

Evelyne Lemaire
Head of Compliance EUCAN, Takeda Pharmaceuticals International GmBh, Zürich und Umgebung, Schweiz |

Natasha Leskovsek, RN, MPM, MBA, JD
Health Care & Life Sciences Regulatory Practice, Cooley, LLP, Washington, DC |

John Linehan, Esq.
Healthcare Attorney, Epstein Becker Green, Washington, DC
|

Michael K. Loucks, JD
Partner, Skadden Arps LLP; Former Acting United States Attorney, District of Massachusetts, United States Department of Justice, Washington, DC |

David J. Ludlow, JD
Partner, Sidley Austin LLP, Washington, DC |

Seth Lundy, Esq.
Partner, King and Spalding, Washington, DC
|

Jill Mason, JD
Chief Compliance Officer, OrthoFix, Former Senior Global Compliance Director, St. Jude Medical, Dallas, TX |

Heather McCollum, JD, MHA
Director, Compliance, Shionogi, Inc., Florham Park, NJ |

Maureen McGirr, Esq.
Vice President, Office of Ethics, Global Compliance Organization, Merck & Co., Inc., Kenilworth, NJ
|

Jennifer McGee, Esq.
Chief Compliance Officer, Otsuka Pharmaceutical Development & Commercialization, Inc., Rockville, MD |

Chris Morris, MBA, CPA, CFE
Managing Consultant, Healthcare & Life Sciences Disputes, Regulatory, Compliance & Investigations, Navigant, Phoenix, AZ |

John Murphy, JD
Assistant General Counsel, Pharmaceutical Research and Manufacturers of America (PhRMA), Washington, DC
|

Chrisoula Nikidis
Executive Director, Ethics and Compliance, Innovative Medicines Canada; Industry Co-Chair Designate, APEC Biopharmaceutical Working Group on Ethics, Ottawa, Canada |

Michael O'Connor
Senior Director, Ethics and Compliance Operations, Alexion Pharmaceuticals, Inc., Cheshire, CT |

William P. Olsen
Principal, Practice Leader, Corporate Compliance, Forensic Advisory Services, Grant Thornton LLP, McLean, VA
|

John Patrick Oroho, Esq.
Executive Vice President and Chief Strategy Officer, Porzio Life Sciences, LLC, Principal, Porzio, Bromberg & Newman PC, Morristown, NJ |

Mohammad Ovais
Founder and Chief Executive Officer, qordata, Houston, TX |

Amy Pawloski, CCEP, PMP
Head of US Compliance and Ethics Monitoring and Data Analytics, Bristol-Myers Squibb, Plainsboro, NJ
|

Marie-Claire Pickaert
Deputy Director General and Chief Financial Officer, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium |

Mario Prohasky
Senior Manager, Polaris, New York, NY |

John Rah, JD
Partner, Morgan, Lewis & Bockius LLP, Washington, DC
|

Kelly N. "Nikki" Reeves, MPA, JD
Partner, King & Spalding LLP, Washington, DC |

Francisco Ribeiro, LLB, MBA
Senior Director and Corporate Compliance Officer, Biogen, Cambridge, MA |

Kevin Ryan, JD, MS
Senior Director, Compliance: New Products, Novo Nordisk, Princeton, NJ
|

Linda Sarnecki
Director, Business Partner Transparency and Compliance, Sanofi US, New York, NY |

Sue Seferian, Esq.
Global R&D Health Care Compliance Officer, Johnson & Johnson, New Brunswick, NJ |

Himani Shah
Senior Director, Global Compliance Department, AstraZeneca, Gaithersburg, MD
|

John Shakow, JD
Partner, FDA and Life Sciences Practice, King & Spalding, Washington, DC |

Brian Sharkey, JD
Vice President, Porzio Life Sciences, LLC, Morristown, NJ |

Michelle Shwery, MBA
Senior Advisor, Ethics and Compliance, Eli Lilly and Company, Indianapolis, IN
|

Steven Sitek, Med
Ethics & Compliance Learning & Education, Novartis Pharmaceuticals Corporation, East Hanover, NJ |

Jon Smollen, JD, MA
Director, Temple Law Center for Compliance and Ethics; Former Executive Vice President and Chief Compliance Officer, Endo International, Philadelphia, PA |

Don Soong
General Manager, Transparency, QuintilesIMS, Warren, NJ
|

James Stansel, Esq.
EVP and General Counsel, PhRMA, Former Acting General Counsel, U.S. Department of Health and Human Services, Washington, DC |

Jack Tanselle, MBA
Managing Director, Huron Life Sciences, Indianapolis, IN |

Lee Taurman
Principal, National Life Sciences Advisory Leader, Grant Thornton LLP, Iselin, NJ
|

Bryan Timer, MIS
Associate Director, Data Analytics & Transparency, Merck & Co Inc., Lansdale, PA |

Michael Trahar
Senior Manager - Assurance Services, EY, Washington DC |

Nancy Schwalje Travis
Vice President, International Compliance and Governance, Advanced Medical Technology Association (AdvaMed), Washington, DC
|

Seth B. Whitelaw, JD, PhD
President & Chief Executive Officer, Whitelaw Compliance Group, LLC, Editor, Life Science Compliance Update, West Chester, PA |

Ronald Wisor, Esq.
Partner, Hogan Lovells, Washington, DC |

Alexis Wong
Director, PwC Risk Consulting, Denver, CO
|

Doug Worthington, JD
VP, Compliance & Ethics, US, Bristol-Myers Squibb, Princeton, NJ |

Lisa Zavis, MBA
Director, Data Analytics & Transparency, Merck & Co Inc., Kenilworth, NJ
|
|
|
|
INVITATION TO ATTEND THE PCF PHARMA CONGRESS
|
|
 As Vice President, Pharma and Medical Device Content, Global Health Care - Life Sciences, LLC, it is my pleasure to invite you to attend the 2016 Seventeenth Annual Pharmaceutical and Medical Device Compliance Congress and Best Practices Forum sponsored by the PCF. This year's Congress will feature presentations by leading government regulators, company compliance professionals, in-house counsel, prominent industry consultants and legal counsel. Please plan to join me and the Planning Committee for three stimulating days of continuing education, networking and best practice sharing.
As Vice President, Pharma and Medical Device Content, Global Health Care - Life Sciences, LLC, it is my pleasure to invite you to attend the 2016 Seventeenth Annual Pharmaceutical and Medical Device Compliance Congress and Best Practices Forum sponsored by the PCF. This year's Congress will feature presentations by leading government regulators, company compliance professionals, in-house counsel, prominent industry consultants and legal counsel. Please plan to join me and the Planning Committee for three stimulating days of continuing education, networking and best practice sharing.
Kelly B. Freeman, PhD
Vice President, Pharma and Medical Device Content, Global Health Care - Life Sciences, LLC, Former Senior Advisor, Ethics and Compliance, Eli Lilly and Company, Former Member, PCF Executive Committee, Indianapolis, IN
|
|
|
|
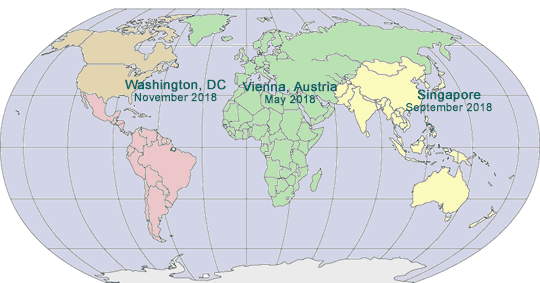
2016-2017 GLOBAL PHARMA COMPLIANCE CONGRESSES
|
|
SIXTH ASIA PACIFIC
PHARMACEUTICAL AND
MEDICAL DEVICE
COMPLIANCE CONGRESS
|
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by International Society of Healthcare Ethics and Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
September 21 - 23, 2016
Shangri-La Hotel
Singapore
www.AsianPharma
Congress.com
|
|
SEVENTEENTH ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum
October 19 - 21, 2016
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
|
|
ELEVENTH INTERNATIONAL
PHARMACEUTICAL
COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Cosponsored by Pharmaceutical Compliance Forum (PCF)
May 15 - 17, 2017
Lisbon Marriott Hotel
Lisbon, Portugal
www.International
PharmaCongress.com
|
|
EIGHTEENTH ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum
November 6 - 8, 2017
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
|
|
|
OTHER GLOBAL PHARMA CONGRESS CITIES
|

2007 - Brussels

2008 - Paris

2009 - Rome

2010 - Berlin
|

2011 - Istanbul

2011 - Singapore

2012 - Budapest

2012 - Shanghai
|

2012 - São Paulo

2013 - Madrid

2013 - Kuala Lumpur

2014 - Dubai
|
|

2014 - Mexico City

2014 - Shanghai
|
|

2015 - Manila

2016 - Warsaw
|
|
|
|
|

|
DOWNLOAD THE
CONFERENCE APP
|
|
Click here to download the conference app.
|
|
|
|
SPONSORED BY:
|

The Pharmaceutical Compliance Forum (PCF) is a coalition of senior compliance professionals and legal counsel from almost 60 research-based pharmaceutical manufacturers. The PCF was founded in early-1999 by compliance professionals from the pharmaceutical industry to promote effective corporate compliance programs. The members meet several times a year, focusing on open and informal sharing of compliance information, best practices, and current developments in the field. PCF also sponsors this three-day compliance congress each Fall.
|
|
CO CHAIRS
|

Timothy Ayers, JD, MPH
Vice President, Chief Compliance Officer, Horizon Pharma plc, Chicago, IL
|

Joseph Boyd
Director, Commercial Planning & Operations, Primus Pharmaceuticals, Inc., Scottsdale, AZ
|

Matthew D'Ambrosio, JD, MBA
Senior Vice President, Chief Compliance and Ethics Officer, Sunovion Pharmaceuticals Inc., Marlborough, MA
|

James Gibney
Senior Director of Compliance, Regeneron Pharmaceuticals, Tarrytown, NY
|

Jeffrey Kawalek, MBA
Associate Director, Compliance Risk, Novo Nordisk, New York, NY
|

Glenna Shen, JD, MBT
Executive Director, Worldwide Compliance and Business Ethics, Amgen Inc., Thousand Oaks, CA
|
|
|
GRANTORS:
|
|
GOLD
|

|
|
SILVER
|





|
|
BRONZE
|













|
|
MEDIA PARTNERS:
|



|
|
CONTINUING EDUCATION CREDITS
|
Accounting Professionals: Approved for up to 16.50 NASBA CPE credits.
Compliance Professionals: The Congress has been approved for 22.5 Compliance Certification Board CCB Credits.
Attorneys: The Congress has been approved for up to 14.00 Pennsylvania MCLE Credits.
Click here for more information.
|
|
2016 PCF PHARMA CONGRESS PLANNING COMMITTEE
|
Timothy Ayers, JD, MPH, Vice President, Chief Compliance Officer, Horizon Pharma plc; Former Vice President, Chief Compliance Officer, Dendreon, Chicago, IL (Co-chair)
Joseph Boyd, Director, Commercial Planning & Operations, Primus Pharmaceuticals, Inc.; Former Director, Operations, Ethics & Compliance Department, Astellas US LLC, Scottsdale, AZ (Co-chair)
Matthew D'Ambrosio, JD, MBA, Senior Vice President, Chief Compliance and Ethics Officer, Sunovion Pharmaceuticals Inc., Marlborough, MA (Co-chair)
James Gibney, Senior Director of Compliance, Regeneron Pharmaceuticals; Former Director, Worldwide Programs and US Investigations, Corporate Compliance, Pfizer, Tarrytown, NY (Co-chair)
Jeffrey Kawalek, MBA, Associate Director, Compliance Risk, Novo Nordisk, New York, NY (Co-chair)
Glenna Shen, JD, MBT, Executive Director, Worldwide Compliance and Business Ethics, Amgen Inc., Thousand Oaks, CA (Co-chair)
Eric Baim, Vice President, Head of Compliance US, Shire, Boston, MA
Scott Bass, JD, Partner and Head, Global Life Sciences Team, Sidley Austin LLP, Washington, DC
John T. Bentivoglio, JD, Partner, Skadden Arps LLP, Washington, DC
Yogesh Bahl, CPA, MBA, Managing Director, Alix Partners, New York, NY
Ann Beasley, JD, Director, Navigant; Former Senior Vice President, Chief Compliance Officer, Biogen, Boston, MA
Thomas Beimers, JD, Partner, Hogan Lovells, Minneapolis, MN
Andy Bender, MS, MBA, President and Founder, Polaris, New York, NY
Michael Clarke, JD, Vice President, Corporate Compliance, Indivior, Richmond, VA
Clarissa Crain, Senior Manager, Life Sciences & Health Care, Deloitte & Touche LLP, Philadelphia, PA
Kris Curry, Principal, Fraud Investigation and Dispute Services, EY, Philadelphia, PA
Michael B. Dusseau, Vice President, Compliance Operations, Allergan plc, Parsippany, NJ
Margaret K. Feltz, MA, JD, Executive Director, Acting Chief Compliance Officer, Ethics & Compliance, Purdue Pharma LP, Stamford, CT
Kellie B. Combs, JD, Counsel, Ropes & Gray, Chicago, IL
Jeffrey Fleming, JD, Vice President and Chief Compliance Officer, Vertex Pharmaceuticals; Former Vice President Compliance North America and US Compliance Officer, AstraZeneca Pharmaceuticals LP, Boston, MA
Gary F. Giampetruzzi, JD, Partner, Paul Hastings, New York, NY
Virginia “Ginny” A. Gibson, JD, Partner, Hogan Lovells LLP, Philadelphia, PA
Wendy C. Goldstein, JD, Partner, Health Care and Life Sciences Regulatory Practice, Cooley, LLP, New York, NY
Steven Guymon, Senior Compliance Advisor, Eli Lilly and Company, Indianapolis, IN
Simone Handler-Hutchinson, JD, Assistant Dean for Graduate & Professional Education, Seton Hall Law, Newark, NJ
Erinn Hutchinson, Partner, Advisory Services, PwC, Philadelphia, PA
Jeffrey S. Klimaski, MBA, CPA, Vice President, Global Ethics and Compliance Officer, BTG International Inc., Philadelphia, PA
Keith M. Korenchuk, JD, MPH, Partner, Arnold & Porter LLP, Washington, DC
Daniel A. Kracov, JD, Partner and Head, FDA and Healthcare Practice, Arnold & Porter, Washington, DC
Terri Ledva, MS, Ethics & Compliance Leader, Iroko Pharmaceuticals, Philadelphia, PA
Christine Longawa, MA, CPA, CFE, Life Sciences Disputes, Compliance and Investigations Associate Director, Navigant Consulting, Chicago, IL
Jill Mason, JD, Chief Compliance Officer, Orthofix, Dallas, TX
Maureen McGirr, JD, Vice President Office of Ethics, Global Compliance Organization, Merck, Philadelphia, PA
MaryAnn Northrup, CFE, CHRC, Senior Director, FTI Consulting, Indianapolis, IN
Ed Nowicki, JD, Vice President and General Counsel, Pfizer, Inc., New York, NY
John Patrick Oroho, JD, Executive Vice President, and Chief Strategy Officer, Porzio Life Sciences, LLC; Principal, Porzio, Bromberg & Newman PC, Morristown, NJ
Lori Queisser, Senior Vice President and Global Chief Compliance Officer, Teva Pharmaceuticals, Horsham, PA
Arjun Rajaratnam, JD, MS, Chief Compliance Officer, Smith & Nephew, Raleigh, NC
David Ralston, JD, MPH, Senior Director, Associate General Counsel, Business Conduct, Gilead Sciences, Foster City, CA
Lauren K. Roth, JD, Assistant General Counsel, PhRMA, Washington, DC
Kelly N. “Nikki” Reeves, MPA, JD, Partner, King & Spalding LLP, Washington, DC
Michael Shaw, Esq. Vice President and Compliance Officer, US Pharmaceuticals, GlaxoSmithKline, Philadelphia, PA
Karen Sheehy, JD, Vice President, Head of North America Compliance, Sanofi, Bridgewater, NJ
Paul Silver, Practice Leader and Managing Director, Huron Consulting Group, Atlanta, GA
Carlos Tessi, MD, PhD, Vice President, Compliance, Shionogi, Inc., Florham Park, NJ
Donna Wachman, National Industry Marketing Leader, Grant Thornton LLP, Charlotte, NC
Seth B. Whitelaw, JD, PhD, President and Chief Executive Officer, Whitelaw Compliance Group, LLC; Editor, Life Science Compliance Update, West Chester, PA
|
FOLLOW PHARMA
CONGRESS ON
|


|
|
PHARMA CONGRESS IS
|

|
|
PARTICIPATION OPTIONS
|
|
|
|
TRADITIONAL ONSITE ATTENDANCE
|
Simply register, travel to the conference city and attend in person.
Pros: subject matter immersion; professional networking opportunities; faculty interaction

|
|
LIVE AND ARCHIVED WEBCAST PARTICIPATION
|
Watch the conference in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office


|
WEBCAST INTERFACE SAMPLE
|
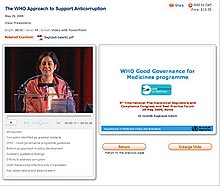
Click here for a sample stream
|
THE 2015 SIXTEENTH ANNUAL PHARMA CONGRESS CONTENT IS NOW AVAILABLE IN VARIOUS POST CONFERENCE FORMATS
|
|
The 2015 Fifteenth Annual Pharma Congress conference content is now available in a variety of formats.
You may purchase the Congress streaming content in the following formats: Flash Drive or online archive (6 months). You may also purchase individual presentations in an online archive (6 months) format.
YOU CAN PURCHASE JUST THE PHARMA CONGRESS CONTENT AS FOLLOWS:
|

Online Archive of 2015 Sixteenth Annual Pharma Congress Presentations today!
$595
Order Now

Flash Drive of 2015 Sixteenth Annual Pharma Congress Presentations today!
$595
Order Now
|
|
FINALLY YOU MAY PURCHASE PHARMA CONGRESS INDIVIDUAL PRESENTATIONS:
Click here to purchase individual presentations for $59.95 in online archive format (6 months of access - 24/7).
|
|

 This site complies with the HONcode standard for trustworthy health information: This site complies with the HONcode standard for trustworthy health information:
verify here.


|


|
|

|

